Whole Organ Perfusion
Whole Organ Perfusion / Contrast Transport / Cathlab Diagnostics / Permeability Estimation
porcine coronary Vascular structure imaged with imaging cryomicrotome
Vascular Network
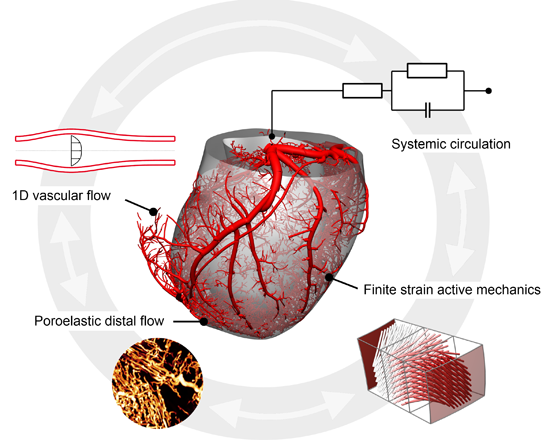
The coronary vasculature has a multiscale organisation. At the proximal end, the large epicardial vessels and intramural arteries direct blood flow to the myocardial territories. The smaller arterioles provide the resting tone and a significant part of the haemodynamic resistance. At the distal end, capillaries address perfusion and nutrient exchange, and the venules and veins serve a capacitance function.
Flow-Contraction Coupling
In addition to intravascular factors, the cardiac contraction imposes a significant impedance to coronary flow and is responsible for the phasic flow velocity observed. The intramyocardial stress is responsible for the systolic flow impediment observed, and is implicated as a potential mechanism contributing to subendocardial vulnerability to myocardial ischemia.
whole organ perfusion model in beating heart
Whole Organ Modelling
Various approaches have been employed to model coronary flow to date, including 3D CFD, 1D network models and lumped parameter approaches. While there have been attempts to study whole-organ blood flow using these models, they are either limited in their ability to represent the spatial heterogeneity of the flow, and/or do not facilitate coupling with cardiac contraction. To address these shortcomings, we have developed an integrated modelling framework which combines flow in individual vessels of the proximal network with a poromechanical representation of the coupled distal network and myocardial mechanics.
The integrated model greatly extends the capability to pursue many previously difficult applications such as coronary wave intensity analysis and regional perfusion evaluation. Using the proposed model, these investigations can be studied in conjunction with various diseases of the myocardium and coronary vessels, characterised directly from non-invasive medical imaging. Work is under way to combine personalised cardiac mechanics with altered myocardial blood flow. In addition, this modelling framework affords novel uses in virtual imaging through biophysical simulation of contrast agent transport, which is outlined here.